Major Histocompatibility Complex and Their Molecules
The Roles of MHC Molecules In Adaptive Immune Responses
MHC molecules enable T-lymphocytes to recognize epitopes of antigens and discriminate self from non-self. Unlike B-cell receptors on B-lymphocytes that are able to directly bind epitopes on antigens, the T-cell receptors (TCRs) of T-lymphocytes can only recognize epitopes - typically short chains of amino acids called peptides - after they are bound to MHC molecules.
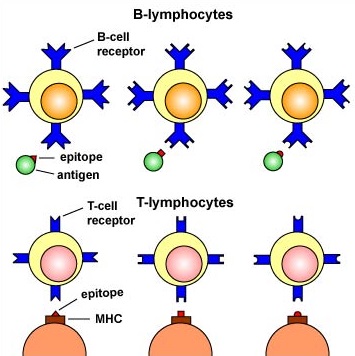
Epitope-Specific Receptors on the surface of B- and T-Lymphocytes. B-lymphocytes have B-cell receptors that recognize epitopes directly on antigens. T-lymphocytes have TCR molecules that recognize epitopes only after they have been placed on cells of the body by way of MHC molecules.
The MHC genes are the most polymorphic genes in the human genome, possessing many alleles for each gene. The MHC genes are co-dominantly expressed so that an individual expresses the alleles inherited from each parent. In this way, the number of MHC molecules that bind peptide for presentation to T-lymphocytes is maximized. In addition, each MHC molecule is able to bind a wide variety of different peptides, both self-peptides, and foreign peptides. There are two classes of MHC molecules: MHC-I and MHC-II.
- MHC-I molecules present epitopes to T8-lymphocytes.
- MHC-II molecules present epitopes to T4-lymphocytes.
The expression of MHC molecules is increased by cytokines produced during both innate immune responses and adaptive immune responses. Cytokines such as interferon-alpha, interferon-beta, interferon-gamma, tumor necrosis factor increase the expression of MHC-I molecules, while interferon-gamma is the main cytokine to increase the expression of MHC-II molecules.
MHC-I molecules
MHC-I molecules are designed to enable the body to recognize infected cells and tumor cells and destroy them with cytotoxic T-lymphocytes or CTLs. CTLs are effector defense cells derived from naive T8-lymphocytes. MHC-I molecules are:
- Made by all nucleated cells in the body.
- Possess a deep groove that can bind peptide epitopes, typically 8-11 amino acids long, typically from endogenous antigens.
- Present MHC-I/peptide complexes to naive T8-lymphocytes and cytotoxic T-lymphocytes possessing a complementary-shaped T-cell receptor or TCR.
- Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of exogenous antigens to MHC-I molecules for eventual presentation to naive T8-lymphocytes.
Endogenous antigens are proteins found within the cytosol of human cells. Examples of endogenous antigens include:
a. Viral proteins produced during viral replication;
b. Proteins produced by intracellular bacteria such as Rickettsias and Chlamydias during their replication;
c. Proteins that have escaped into the cytosol from the phagosome of phagocytes such as antigen-presenting cells;
d. Tumor antigens produced by cancer cells; and
e. Self-peptides from host cellular proteins.
During the replication of viruses and intracellular bacteria within their host cell, as well as during the replication of tumor cells, viral, bacterial, or tumor proteins are degraded into a variety of peptide epitopes by cylindrical organelles called proteasomes. The body's own cytosolic proteins are also degraded into peptides by proteasomes.
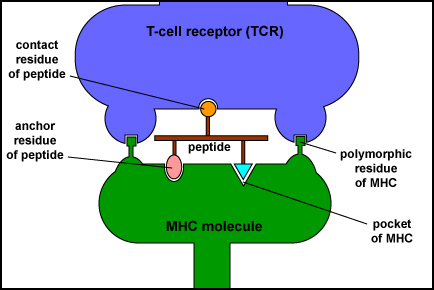
MHC Molecule with Bound Peptide Binding to a Complementary T-Cell Receptor. This illustration shows a T-cell receptor (TCR) on the surface of a T-lymphocyte recognizing two polymorphic residues of an MHC molecule and one residue of its bound peptide epitope.
These peptide epitopes are then attached to a groove of MHC-I molecules that are then transported to the surface of that cell where they can be recognized by a complementary-shaped T-cell receptor (TCR) and a CD8 molecule, a co-receptor, on the surface of either a naive T8-lymphocyte or a cytotoxic T-lymphocyte (CTL). The TCRs recognize both the foreign peptide antigen and the MHC molecule. TCRs, however, will not recognize self-peptides bound to MHC-I. As a result, normal cells are not attacked and killed.
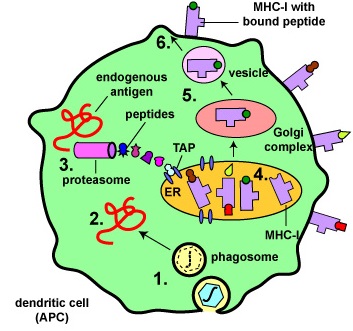
Binding of Peptide Epitopes from Endogenous Antigens to MHC-I Molecules by a Dendritic Cell. Endogenous antigens are those located within the cytosol of the cells of the body. Examples include a. viral proteins produced during viral replication, b. proteins produced by intracellular bacteria such as Rickettsias and Chlamydias during their replication, c. proteins that have escaped into the cytosol from the phagosome of phagocytes such as antigen-presenting cells, d. tumor antigens produced by cancer cells, e. and self-peptides from human cell proteins.
Dendritic cells bind epitopes from endogenous antigens to MHC-I molecules and present them to naive T8-lymphocytes in order to activate these naive T8-lymphocytes.
- Antigens are engulfed by dendritic cells and placed in a phagosome. Some of the proteins escape from the phagosome into the cytosol of the dendritic cell where they become endogenous antigens.
- These endogenous antigens pass through proteasomes where they are degraded into a series of peptides.
- The peptides are transported into the rough endoplasmic reticulum (ER) by a transporter protein called TAP.
- The peptides then bind to the grooves of newly synthesized MHC-I molecules.
- The endoplasmic reticulum transports the MHC-I molecules with bound peptides to the Golgi complex.
- The Golgi complex, in turn, transports the MHC-I/peptide complexes by way of an exocytic vesicle to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-I/peptide complexes can be recognized by naive T8-lymphocytes by way of TCRs and CD8 molecules having a complementary shape.
Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of exogenous antigens to MHC-I molecules for eventual presentation to naive T8-lymphocytes.
MHC-I molecule with bound peptide on the surface of antigen-presenting dendritic cells can be recognized by a complementary-shaped TCR/CD8 on the surface of a naive T8-lymphocyte to initiate cell-mediated immunity. (Certain dendritic cells, as discussed later, can also cross-present exogenous antigens to MHC-I molecules).
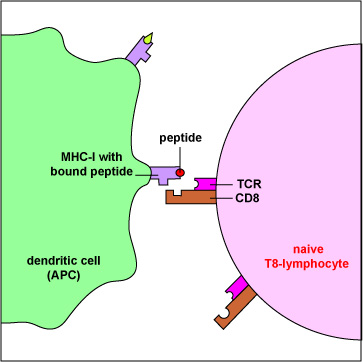
An Antigen-Presenting Dendritic Cell Presenting MHC-I with Bound Peptide to a Naive T8-lymphocyte having a Complementary T-Cell Receptor. Antigen-presenting dendritic cells produce both MHC-I and MHC-II molecules. These APCs can phagocytose infected cells and tumor cells, place them in phagosomes, and degrade them with lysosomes. During this process, some of the proteins escape from the phagosome into the surrounding cytosol. Here they can be degraded into peptides by proteasomes, bound to MHC-I molecules, and placed on the surface of the dendritic cell. Now the peptide/MHC-I complexes can be recognized by a naive T8-lymphocyte having a complementary-shaped T-cell receptor (TCR) and CD8 molecule. This activates the naive T8-lymphocyte enabling it to eventually proliferate and differentiate into cytotoxic T-lymphocytes (CTLs).
MHC-I molecule with bound peptide on the surface of infected cells and tumor cells can be recognized by a complementary-shaped TCR/CD8 on the surface of a cytotoxic T-lymphocyte or CTL to initiate the destruction of the cell containing the endogenous antigen. (CTLs are effector cells derived from naive T8-lymphocytes.)
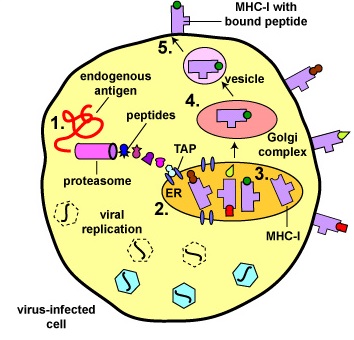
Binding of Peptide Epitopes from Endogenous Antigens to MHC-I Molecules by a Virus-Infected Cell. Endogenous antigens are those being produced within the cytosol of the cells of the body. Examples include a. viral proteins produced during viral replication, b. proteins produced by intracellular bacteria such as Rickettsias and Chlamydias during their replication, c. proteins that have escaped into the cytosol from the phagosome of phagocytes such as antigen-presenting cells. tumor antigens produced by cancer cells, e. and self-peptides from human cell proteins. The body marks infected cells and tumor cells for destruction by placing peptide epitopes from these endogenous antigens on their surface by way of MHC-I molecules.
Cytotoxic T-lymphocytes (CTLs) are then able to recognize peptide/MHC-I complexes by means of their T-cell receptors (TCRs) and CD8 molecules and kill the cells to which they bind.
- During viral replication within the host cell, endogenous antigens, such as viral proteins, pass through proteasomes where they are degraded into a series of peptides.
- The peptides are transported into the rough endoplasmic reticulum (ER) by a transporter protein called TAP.
- The peptides then bind to the grooves of newly synthesized MHC-I molecules.
- The endoplasmic reticulum transports the MHC-I molecules with bound peptides to the Golgi complex.
- The Golgi complex, in turn, transports the MHC-I/peptide complexes by way of an exocytic vesicle to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-I/peptide complexes can be recognized by CTLs by way of TCRs and CD8 molecules having a complementary shape.
MHC-I molecules are coded for by three MHC-I genes, HLA-A, HLA-B, and HLA-C. As mentioned above, however, there are many different alleles for each gene that a person inherits. In this way, the number of MHC-I molecules that bind peptides for presentation to T-8 lymphocytes is maximized. The expression of MHC-I molecules on all cell types is increased by the cytokines interferon-alpha (IFN-a) and interferon-beta (IFN-ß).
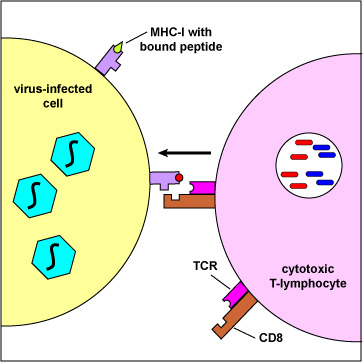
A Cytotoxic T-lymphocyte Recognizing a Virus-Infected Cell. Endogenous antigens are those being produced within the cytosol of the cells of the body. Examples include proteins from replicating viruses, proteins from intracellular bacteria, and tumor antigens. The body marks infected cells and tumor cells for destruction by placing peptide epitopes from these endogenous antigens on their surface by way of MHC-I molecules. Cytotoxic T-lymphocytes (CTLs) are then able to recognize peptide/MHC-I complexes by means of their T-cell receptors (TCRs) and CD8 molecules and kill the cells to which they bind.
All nucleated cells produce MHC-I molecules. MHC-I molecules bind peptide epitopes of antigens found within our cells. Peptide epitopes bound to MHC-I molecules are recognized by TCRs and CD8 molecules on the surfaces of naive T8-lymphocytes and on cytotoxic T-lymphocytes (CTLs).
Why is it important that all nucleated cells in our body are able to produce MHC-I molecules?
MHC-II molecules
MHC-II molecules are designed to enable T4-lymphocytes to recognize epitopes of exogenous antigens and discriminate self from non-self. MHC-II molecules are:
- Made by antigen-presenting cells or APCs, such as dendritic cells, macrophages, and B-lymphocytes.
- Possess a deep groove that can bind peptide epitopes, often 10-30 amino acids long but with an optimum length of 12-16 amino acids, typically from exogenous antigens. The peptides interact along their entire length with the groove.
- Present MHC-II/peptide complexes to naive T4-lymphocytes or effector T4-lymphocytes that have a complementary-shaped T-cell receptor or TCR.
- Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of endogenous antigens to MHC-II molecules for eventual presentation to naive T4-lymphocytes.
Exogenous antigens are antigens that enter from outside the body, such as bacteria, fungi, protozoa, and free viruses. These exogenous antigens enter macrophages, dendritic cells, and B-lymphocytes through phagocytosis. The microbes are engulfed and placed in a phagosome which then fuses with lysosomes. Following this fusion, the phagolysosome becomes acidified. Acidification, in turn, activates the proteases within the phagolysosome enabling protein antigens from the microbe to be degraded into a series of short peptides. These peptide epitopes are then attached to MHC-II molecules and are then transported to the surface of the antigen-presenting cell (APC). (Certain dendritic cells, as discussed later, can also cross-present endogenous antigens to MHC-II molecules.)
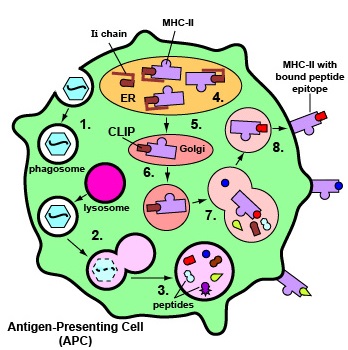
Binding of Peptide Epitopes from Exogenous Antigens to MHC-II Molecules Exogenous antigens are those from outside cells of the body. Examples include bacteria, free viruses, yeasts, protozoa, and toxins. These exogenous antigens enter antigen-presenting cells or APCs (macrophages, dendritic cells, and B-lymphocytes) through phagocytosis. The microbes are engulfed and placed in a phagosome. After lysosomes fuse with the phagosome, protein antigens are degraded by proteases into a series of peptides. These peptides eventually bind to grooves in MHC-II molecules and are transported to the surface of the APC. T4-lymphocytes are then able to recognize peptide/MHC-II complexes by means of their T-cell receptors (TCRs) and CD4 molecules. 1. Exogenous antigens, such as viruses, are engulfed and placed in a phagosome.2. Lysosomes fuse with the phagosome forming a phagolysosome. 3. Protein antigens are degraded into a series of peptides. 4. MHC-II molecules are synthesized in the endoplasmic reticulum and transported to the Golgi complex. Once assembled, within the endoplasmic reticulum, a protein called the invariant chain (Ii) attaches to the peptide-binding groove of the MHC-II molecules and in this way prevents peptides designated for binding to MHC-I molecules within the ER from attaching to the MHC-II. 5. As the MHC-II molecules with bound Ii chain are transported to the Golgi complex, the Ii is cleaved, leaving a short peptide called CLIP in the groove of the MHC molecule. 6&7. The vesicles containing the MHC-II molecules fuse with the peptide-containing phagolysosomes. The CLIP peptide is removed from the MHC=II molecules and the peptide epitopes are now free to bind to the grooves of the MHC-II molecules. 8. The MHC-II molecules with bound peptides are transported to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-II complexes can be recognized by T4-lymphocytes by way of TCRs and CD4 molecules having a complementary shape. (Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of endogenous antigens to MHC-II molecules for eventual presentation to naive T4-lymphocytes.)
Some pathogens, such as Mycobacterium tuberculosis, Mycobacterium leprae, and Leishmania, are able to grow in the endocytic vesicles of macrophages without being killed by lysosomes. These macrophages can, however, become activated by T4-effector lymphocytes called TH1 cells and subsequently use intravesicular proteases to degrade the proteins from these pathogens into peptides for presentation to MHC-II molecules that pass through on their way to the cell surface.
Here the MHC-II molecules with bound peptides can be recognized by a complementary-shaped T-cell receptor and a CD4 molecule, a co-receptor, on the surface of a T4-lymphocyte. T4-lymphocytes are the cells the body uses to regulate both humoral immunity and cell-mediated immunity.
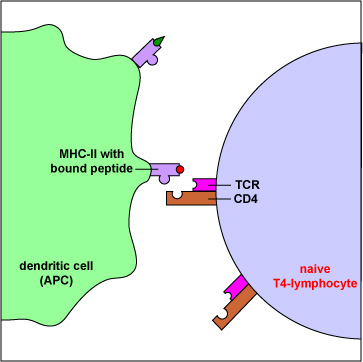
A T4-Lymphocyte Recognizing Epitope/MHC-II on an Antigen-Presenting Dendritic Cell. Exogenous antigens are those from outside cells of the body. Examples include bacteria, free viruses, yeasts, protozoa, and toxins. These exogenous antigens enter antigen-presenting dendritic cells through phagocytosis. The microbes are engulfed and placed in a phagosome. After lysosomes fuse with the phagosome, protein antigens are degraded by proteases into a series of peptides. These peptides eventually bind to grooves in MHC-II molecules and are transported to the surface of the APC. T4-lymphocytes are then able to recognize peptide/MHC-II complexes by means of their T-cell receptors (TCRs) and CD4 molecules.
MHC-II molecules are coded for by three MHC-II genes, HLA-DR, HLA-DP, and HLA-DQ. Interferon-gamma (IFN- ?) increases the expression of both MHC-I and MHC-II molecules.
Think-Pair-Share Questions
Only antigen-presenting cells such as dendritic cells, macrophages, and B-lymphocytes produce MHC-II molecules. Peptide epitopes bound to MHC-II molecules are recognized by TCRs and CD4 molecules on the surfaces of naive T4-lymphocytes and on effector T4-lymphocytes.
Why don't all nucleated cells in our body produce MHC-II molecules as well as MHC-I molecules?
Why is it important for dendritic cells to produce both MHC-I and MHC-II molecules?
Summary
- MHC molecules enable T-lymphocytes to recognize epitopes and discriminate self from non-self.
- T-cell receptors (TCRs) of T-lymphocytes can only recognize epitopes - typically short chains of amino acids called peptides - after they are bound to MHC molecules.
- MHC-I presents epitopes to T8-lymphocytes; MHC-II presents epitopes to T4-lymphocytes.
- MHC-I molecules are designed to enable the body to recognize infected cells and tumor cells and destroy them with cytotoxic T-lymphocytes or CTLs. (CTLs are effector defense cells derived from naïve T8-lymphocytes.)
- MHC-I molecules are made by all nucleated cells in the body; bind peptide epitopes typically from endogenous antigens; present MHC-I/peptide complexes to naive T8-lymphocytes and cytotoxic T-lymphocytes possessing a complementary-shaped T-cell receptor or TCR.
- Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of exogenous antigens to MHC-I molecules for eventual presentation to naive T8-lymphocytes.
- Endogenous antigens are proteins found within the cytosol of human cells and include viral proteins produced during viral replication, proteins produced by intracellular bacteria, proteins that have escaped into the cytosol from the phagosome of phagocytes such as antigen-presenting cells, and tumor antigens produced by cancer cells.
- During the replication of viruses and intracellular bacteria within their host cell, as well as during the replication of tumor cells, viral, bacterial, or tumor proteins are degraded into a variety of peptide epitopes by cylindrical organelles called proteasomes. The resulting peptide epitopes are then attached to MHC-I molecules that are then transported to the surface of that cell.
- Exogenous antigens are antigens that enter from outside the body such as bacteria, fungi, protozoa, and free viruses.
- MHC-II molecules are made by antigen-presenting cells or APCs, such as dendritic cells, macrophages, and B-lymphocytes; bind peptide epitopes typically from exogenous antigens, and present MHC-II/peptide complexes to naive T4-lymphocytes or effector T4-lymphocytes that have a complementary-shaped T-cell receptor or TCR.
- Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of endogenous antigens to MHC-II molecules for eventual presentation to naive T4-lymphocytes.
- Exogenous antigens enter antigen-presenting macrophages, dendritic cells, and B-lymphocytes through phagocytosis, and are engulfed and placed in a phagosome where protein antigens from the microbe are degraded by proteases into a series of peptides. These peptides are then attached to MHC-II molecules that are then put on the surface of the APC.


Comments
Post a Comment